You can download the FDA Briefing document on the new vaccine, presumably compiled by Pfizer, here, which is the nearest we have yet to a scientific paper on the vaccine trial used to authorise the vaccine here in Britain.
I picked up a few things from the detail which may be worth discussing. Firstly, it does seems as if the cohort of 40,000 or so was pretty well selected, and because it was international, gives a reasonable spread across different “disease theatres” in both hemispheres. One odd thing is that the protocol seems to exclude participants with evidence of COVID-19 before 7 days after the first vaccine dose, whereas some of the results seem to include those with previous infection (which they use to waffle about the possibility of preventing re-infection, a phenomenon which has only been recorded in a handful of cases worldwide anyway).
The second point is that if our aim is to prevent the spread of the virus, the trial accepts that it has presented no evidence for this, and must await epidemiological studies in the field once many more have been vaccinated. Neither does the the trial demonstrate the prevention of asymptomatic infection, which relates importantly to the previous point because much stress seems to have been placed, rightly or wrongly, on the role of asymptomatic super-spreaders in the epidemiology of COVID.
The one thing the document does stress is the much-trumpeted “95% reduction in COVID symptoms.” This is the carrot offered to the British public, at least, for receiving vaccination. What it actually seems to mean is “reduction of number of symptomatic COVID patients,” as the data gives no way of saying that vaccinated people in general will get fewer symptoms if they catch the virus subsequently.
Now, since the incidence of severe COVID-19 was very low, let’s see what they mean by “COVID symptoms.” In fact, they relied on incredibly lax criteria for diagnosis: any one from a list of “respiratory infection” symptoms would prompt a PCR test, and a positive test constituted a diagnosis. You will already know the shortcomings of PCR test false positives. The list of symptoms for the “primary endpoints” of the trial were: Fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea or vomiting. For the “secondary endpoints” CDC-listed symptoms of fatigue, headache, nasal congestion or runny nose and nausea. So a snuffle and a false PCR positive would constitute a valid COVID infection data-point in this trial.
If we ignore the false-positives, though, then for the Primary evaluation, ie >7 days after the second dose, 8 in 17,411 got COVID from the vaccine group, and 162 in 17,511 from the placebo group. Hence the “95% effectiveness.” That means that a 0.925% risk of one or more mild COVID symptoms was reduced to a 0.045% risk. That is, over 99% of people got no COVID symptoms in either group, which is nice…
…Until you remember the percentage of vaccinees who got short-term side effects almost identical to the trial’s COVID symptom list:
The most common solicited adverse reactions were injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%); severe adverse reactions occurred in 0.0% to 4.6% of participants, were more frequent after Dose 2 than after Dose 1, and were generally less frequent in participants ≥55 years of age (≤ 2.8%) as compared to younger participants (≤4.6%).
The percentages show that nearly everyone vaccinated got symptoms, a large majority got multiple symptoms, and moreover they often got them twice – after both doses. To exchange a <1% risk of symptoms to a near 100% one doesn’t seem that good a deal!
Symptom prevention, then, is not a reason to get vaccinated. So we really need to consider the effectiveness of the vaccine in preventing serious COVID-19, which the trial defines, not too unreasonably, thus:
For another secondary endpoint, the case definition for a severe COVID-19 case was a confirmed COVID-19 case with at least one of the following:
Clinical signs at rest indicative of severe systemic illness (RR ≥30 breaths per minute, HR ≥125 beats per minute, SpO2 ≤93% on room air at sea level, or PaO2/FiO2 <300 mm Hg);
Respiratory failure (defined as needing high-flow oxygen, noninvasive ventilation, mechanical ventilation, or ECMO);
Evidence of shock (SBP <90 mm Hg, DBP <60 mm Hg, or requiring vasopressors);
Significant acute renal, hepatic, or neurologic dysfunction;
Admission to an ICU;
Death.
Now when I was working as a GP, I found that one good way of working through the hype of things we were recommended to do in preventive medicine was to work out the “number needed to treat.” For example, when a patient with a certain risk profile learned that it would need 160 people like him to be on statins for life to prevent one heart attack, the impressive figures for risk reduction seemed less impressive, in comparison to being permanently medicalised.
In this trial, the “number needed to treat” to prevent one death was something less than 1 in 20,000, because nobody died of COVID in the control group of that size. In QUALY terms, at £15 a shot, that’s an indeterminate amount more than £600,000 to save one life, well outside the usual NICE criteria for interventions.
So let’s turn to those with severe symptoms short of death, which are represented in this table:
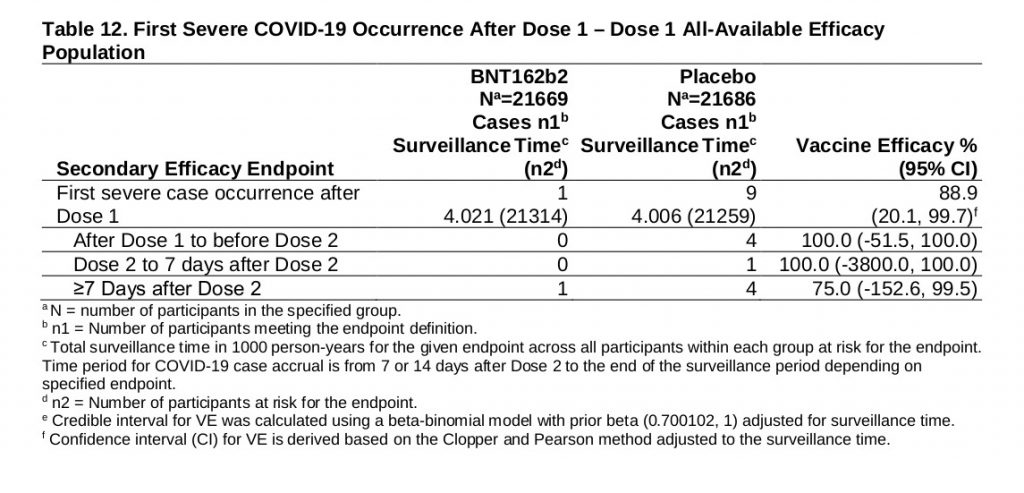
As you see the grand total is 10, and since 9 of these were in the placebo group, the study again claims a 90% prevention rate before it adds provisos. But I can add my own proviso, that even if these total figures were meaningful, they represent a risk of non-fatal, serious COVID, without vaccination, of only 1 in 2,500.
But they are, in fact, clearly less than meaningful (as parts of the paper acknowledge). Consider how this vaccine works: it introduces viral-spike RNA into our cells, which are then fooled into producing viral proteins, to which our immune system reacts as it would to an infection. All this takes a finite time, as any of us who have had to twiddle our thumbs before sending patients off for viral antibody titres will know. Therefore the chances of the vaccine having mounted an effective antibody response in the fortnight between doses must be very low, and surely inadequate to give a 90% reduction in infections. Another explanation is likely.
I venture to suggest that we must consider how COVID-19 actually occurs in geographical hot-spots, and especially within family groups. We have no way to determine that this small sample of sick people was distributed in any way evenly. One or two disease clusters that happened to pick on placebo-group victims, perhaps with particularly high risk profiles, could easily skew results to seem significant when they are not. I hear that researchers have been surprised at the unexpected effectiveness of the vaccine so soon after the first dose, and have suggested some general inflammatory process as the reason. It seems to me that statistical artifact may be equally plausible on this data.
The Primary endpoint of the trial was aimed at >7 days after the second dose, when full immunity could be expected. Here the data for serious COVID is far less encouraging, and even more statistically suspect. As the table above shows, here we have only 4 serious COVID cases, distributed 3:1 placebo:vaccinated. Here a 0.019% risk has been, apparently, reduced to a 0.005% risk. That’s certainly “75% efficacy,” but if my maths is right the “number needed to treat” to prevent one serious case is 7,105. Which is around £107,000 per non-fatal case prevented.
But with such small numbers, once more the statistical significance must be in doubt. Elsewhere in the paper a similar number of Bell’s Palsy cases in the vaccine group is considered to be consistent with the rate in the general population, rather than an effect of the vaccine. The rate of serious COVID cases varies across populations by just as much: who knows if three placebo-group folks happened to get ill in a higher risk area, and one vaccine-taker in a relatively low risk area? The data doesn’t tell us, and the numbers are so low as to question statistical analysis.
Furthermore, there were two “weird” suspected cases to add to the 4 serious “confirmed” (by PCR) cases above, as a “curious footnote” in the paper shows:
Two serious cases of suspected but unconfirmed COVID-19 were reported, both in the vaccine group, and narratives were reviewed. In one case, a 36-year-old male with no medical comorbidities experienced fever, malaise, nausea, headache and myalgias beginning on the day of Dose 2 and was hospitalized 3 days later for further evaluation of apparent infiltrates on chest radiograph and treatment of dehydration. A nasopharyngeal PCR test for SARS-CoV-2 was negative on the day of admission, and a chest CT was reported as normal.
The participant was discharged from the hospital 2 days after admission. With chest imaging findings that are difficult to reconcile, it is possible that this event represented reactogenicity following the second vaccination, a COVID-19 case with false negative test that occurred less than 7 days after completion of the vaccination series, or an unrelated infectious process. In the other case, a 66-year-old male with no medical comorbidities experienced fever, myalgias, and shortness of breath beginning 28 days post-Dose 2 and was hospitalized one day later with abnormal chest CT showing a small left-sided consolidation. He was discharged from the hospital 2 days later, and multiple nasopharyngeal PCR tests collected over a 10-day period beginning 2 days after symptom onset were negative. It is possible, though highly unlikely, that this event represents a COVID-19 case with multiple false negative tests that occurred more than 7 days after completion of the vaccination regimen, and more likely that it represents an unrelated infectious process.
Whatever the explanation for these cases, they were “serious” problems in the vaccination group that go a long way to counterbalancing the serious COVID cases in the placebo group.
All in all there is plenty here for the cynic to get himself thrown off social media for. Prevention of rare mild COVID symptoms is massively outweighed by side effects of similar type; prevention of even rarer serious COVID is statistically suspect and is scarcely worth the huge cost, which is also true for preventing deaths. The ability to prevent transmission has not yet been demonstrated, and the rushed protocols have not enabled any assessment of long-term side-effects, the most concerning of which, in theory, would result from re-exposure to the virus after a period of time, or when you’re trying to get pregnant, if fears about placental auto-immunity have any substance.
The paper stresses the importance of continuing the trials blind to pick up rare, but serious side effects, but adds that several of the pharmaceutical companies intend to give their placebo groups the vaccine after a few months. This, of course, will make the detection of serious problems that much harder – which is good for the vaccine profits and government compensation coffers, if not for the public good.
Meanwhile, in the overall response to the pandemic, cheap non-pharmaceutical interventions to improve immune health have been addressed only in a perfunctory manner (like Vitamin D supplements) or actively suppressed (by locking the vulnerable away from sunlight, exercise and the Coronaviruses that could produce cross-immunity to SARS-CoV-2). Also suppressed (apparently by the clandestine efforts of Big Pharma to discredit them) have been relatively cheap and familiar, and increasingly validated, therapeutic interventions like Ivermectin and chloroquine/zinc/azithromycin.
All this assumes, perhaps against current evidence, that COVID-19 is going to remain a significant threat now it appears to be becoming endemic and seasonal. Now roll up your sleeve and fetch the paracetamol.
